Weekly Quick Hits (BioHealth Capital Region) – Week of May 22, 2023
By Sarah Ellinwood
May 26, 2023
Funding, Awards and Collaborations
Adaptive Phage Therapeutics Partners with Hebrew University, Hadassah Medical Center
Adaptive Phage Therapeutics forged a collaboration and license agreement with the Israeli Phage Therapy Center (IPTC) of the Hebrew University of Jerusalem and Hadassah Medical Center. Researchers at the IPTC have discovered several phages that have broad coverage against antibiotic resistant bacteria. Under the agreement, Hadasit and Yissum, the technology transfer companies of Hadassah and the Hebrew University, grants APT exclusive rights to make, use, and sell licensed phages for human therapy, with the right to sublicense through multiple tiers. APT also has the first right to evaluate additional phages discovered by IPTC researchers for inclusion in APT’s phage bank. As consideration for the license and first right of refusal, APT made an upfront payment and will pay royalties from net sales on any therapeutic composition comprising a licensed phage.
HanAll Biopharma and Daewoong Pharmaceutical Enter into Co-Development Agreement with NurrOn Pharmaceuticals to Develop Therapy for Parkinson’s Disease
HanAll Biopharma Co., Ltd. (KRX: 009420.KS) and Daewoong Pharmaceutical Co., Ltd. (KRX: 069620.KS) announced that they have entered into a co-development agreement with NurrOn Pharmaceuticals Inc., a Boston-based preclinical-stage biopharma. NurrOn is focused on the discovering and developing novel therapies for people with neurodegenerative diseases including Parkinson’s disease (PD) by targeting Nurr1, the master regulator for dopaminergic neuron development and maintenance. HanAll and Daewoong also participated in a Series A funding of NurrOn in 2021.
Precision for Medicine Honored in Every Category of 2023 CRO Leadership Awards
Precision for Medicine, the first biomarker-driven clinical research organization (CRO), announced its wide recognition in the 2023 CRO Leadership Awards. The company has been honored in all five categories: Capabilities, Compatibility, Expertise, Quality, and Reliability, showcasing its exceptional performance in the CRO industry. Most notably, Precision for Medicine secured awards in the Overall, Big Pharma, and Small Pharma categories. In addition, the company received the distinguished Champion Awards in the Capabilities, Compatibility, Expertise, Quality, and Reliability categories within the Big Pharma group, and Reliability in the Overall and Small Pharma categories.
Hememics Biotechnologies Closes $2 Million Seed 2 Financing from TEDCO and Qualified Investors to Accelerate Growth
Hememics Biotechnologies, developer of a first-in-class, handheld, multiplexed biosensor platform that can test antibodies, antigens and molecular targets simultaneously, announced the closing of a $2 million Seed 2 financing round. Participants of this round include a strategic investor, existing investors and Maryland Technology Development Corporation (TEDCO).
In the Clinic
Quoin Pharmaceuticals Announces 50% Enrollment in Netherton Syndrome Clinical Trial
Ashburn, Va.-based Quoin Pharmaceuticals announced its open label study evaluating QRX003 as a treatment for Netherton Syndrome has reached 50% enrollment.The study is investigating the safety and efficacy of QRX003 topical lotion in Netherton Syndrome patients who are currently receiving off-label systemic therapy, primarily biologic therapy.
Innovative Cellular Therapeutics (ICT) Presents Data at the American Society of Gene and Cell Therapy (ASGCT) 26th Annual Meeting
Innovative Cellular Therapeutics (ICT), a clinical-stage biotechnology company developing a comprehensive portfolio of chimeric antigen receptor (CAR) T cell therapies for solid tumors, recently presented at the American Society of Gene and Cell Therapy (ASGCT) 26th Annual meeting in Los Angeles, California, May 16-20, 2023. ICT presented GCC19CART, its lead product candidate from the Company’s CoupledCAR® technology, being developed to treat patients with relapsed/refractory metastatic colorectal cancer (R/R mCRC) in an oral presentation.
Vanda Pharmaceuticals Reports Results from a Phase III study of Tradipitant in Motion Sickness
Vanda Pharmaceuticals (Vanda) (Nasdaq: VNDA) shared results from its Phase III study of tradipitant in motion sickness, confirming the previously reported results demonstrating that tradipitant is effective in the prevention of vomiting associated with motion sickness. The Phase III study was conducted in real-world conditions on boats in the coastal waters of the United States. Both 170 mg and 85 mg tradipitant doses were shown to be superior to placebo in preventing vomiting with only 18.3% and 19.5% of participants experiencing vomiting on tradipitant 170 mg and 85 mg respectively, as compared to 44.3% of participants on placebo (p < 0.0001 for both).
Axalbion Presented Promising Phase 2 Clinical Data in Chronic Cough with TRPM8 Agonist AX-8 in an Oral Presentation at the American Thoracic Society (ATS) 2023 International Conference
Axalbion, a clinical-stage biopharmaceutical company developing medicines to treat cough, presented positive results from a Phase 2 proof-of-concept study with its transient receptor potential melastatin 8 (TRPM8) agonist, AX-8, in patients with refractory or unexplained chronic cough, in an oral session at the American Thoracic Society (ATS) 2023 International Conference, held in Washington, DC, from May 19 – 24, 2023. (ClinicalTrials.gov NCT04866563; EudraCT Number 2021-000844-23).
Horizon Therapeutics to Present Data that Advances the Understanding of Rheumatic Diseases at the 2023 EULAR European Congress of Rheumatology
Horizon Therapeutics (Nasdaq: HZNP) today announced plans for the first presentation of data from a Phase 2 study of dazodalibep, the company’s investigational therapy for Sjögren’s syndrome, during the 2023 EULAR European Congress of Rheumatology, May 31 – June 3, 2023, in Milan. The company will also present additional data on KRYSTEXXA® (pegloticase) injection, including new analyses from the MIRROR randomized clinical trial.
Ascentage Pharma Presents Updated Results from Multiple Clinical Studies at American Society of Clinical Oncology Annual Conference
Ascentage Pharma (6855.HK), a global biopharmaceutical company developing therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced that 4 of its abstracts were selected for presentations at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting. These studies report on four of the company’s lead drug candidates, including the first and only China-approved third-generation Bcr-Abl inhibitor, olverembatinib (HQP1351), Bcl-2 selective inhibitor, lisaftoclax (APG-2575), MDM2-p53 inhibitor, alrizomadlin (APG-115), and FAK/ALK/ROS1 inhibitor, APG-2449.
I-Mab to Share Encouraging Phase 1b/2 Study Results of Patients with Advanced NSCLC Receiving Uliledlimab and Toripalimab Combination Therapy at ASCO 2023
I-Mab (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, announced it will be sharing encouraging results from the Phase 1b/2 study (ClinialTrial.gov Identifier: NCT04322006) evaluating uliledlimab, a highly differentiated CD73 antibody, in combination with toripalimab (TUOYI®), a PD-1 antibody, in patients with treatment-naïve advanced non-small cell lung cancer (NSCLC), and exploring the potential value of CD73 expression as a predictive biomarker. The results will be reported in a poster presentation on June 3 at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting.
The study is a dose expansion portion of a Phase 1b/2 trial evaluating the safety and efficacy of the combination therapy and investigating the potential correlation between tumor CD73 expression and clinical response for patients with treatment-naïve advanced NSCLC.
Research Roundup
NexImmune to Present Poster at the 2023 FOCIS Annual Meeting
NexImmune (Nasdaq: NEXI) announced that it will be presenting a poster at the Federation of Clinical Immunology Societies (FOCIS) Annual Meeting, being held in Boston from June 20-23, 2023. This poster is a result of a collaboration between NexImmune and Dr. Steven Jacobsen at the NIH which is focused on two goals. The first is to use NexImmune’s AIM nanoparticle technology to expand and characterize EBV specific CD8+ T cells from healthy and multiple sclerosis (MS) donors to assess whether MS may be associated with defective T cell control of EBV-infected B cells. The second goal is to evaluate whether specific T cell defects can be circumvented as a potential development strategy to enable CD8+ specific killing of EBV+ cells that are believed to be a significant contributing factor to multiple sclerosis.
Regulatory and Advocacy
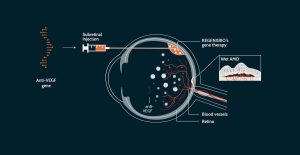
REGENXBIO Receives FDA RMAT Designation for Hunter Syndrome Gene Therapy
REGENXBIO announced the FDA granted Regenerative Medicine Advanced Therapy (RMAT) designation for RGX-121, an investigational one-time AAV Therapeutic for the treatment of Mucopolysaccharidosis Type II (MPS II), also known as Hunter syndrome. RMAT designation is designed to expedite the drug development and review processes for promising new treatments, including gene therapies, and recognizes that the preliminary clinical evidence from RGX-121 indicates its potential to address unmet medical needs for MPS II. RGX-121 is currently being studied in the CAMPSIITE trial.
FDA Approves Indivior’s Opvee Opioid Overdose Rescue Nasal Spray
Richmond, Va.-based Indivior PLC won FDA approval for Opvee (nalmefene) nasal spray for the emergency treatment of known or suspected opioid overdose induced by natural or synthetic opioids in adults and pediatric patients aged 12 years and older. Opvee was approved via the 505(b)(2) pathway. Following Opvee administration, the time to onset of reversal of respiratory depression was observed between 2.5 to 5 minutes and full recovery of respiratory drive was manifested as early as 5 minutes after OPVEE administration. OPVEE contains nalmefene, an opioid receptor antagonist that provides fast onset and long duration reversal of opioid-induced respiratory depression, which is the primary cause of opioid overdose injury and death.
Briefing on Capitol Hill Urges More Government Action and Investment in the Epilepsies
In cooperation with the Congressional Epilepsy Caucus, organizations including the Epilepsy Foundation, Epilepsies Action Network, CURE Epilepsy, Rare Epilepsy Network and DEE-P Connections hosted a briefing on Capitol Hill on May 17 to educate about the epilepsies and call for more government action and investment in the epilepsies. Congressional Epilepsy Caucus co-chairs Congressman Greg Murphy, M.D. (R-NC-3) and Jim Costa (D-CA-21) spoke about their personal connections to epilepsy and the importance of the caucus.
People on the Move
Avalo Appoints Michael Croft and Jeff Edelson to Scientific Advisory Board
Avalo Therapeutics tapped Dr. Michael Croft and Dr. Jeff Edelson for its Scientific Advisory Board. The SAB, which is led by Dr. Carl Ware, provides strategic and scientific guidance to Avalo as it progresses its pipeline of therapies that target the LIGHT-signaling network, notably AVTX-002 (anti-LIGHT mAb) and AVTX-008 (BTLA agonist fusion protein). Croft is Director, Academic Affairs and Professor, Center for Autoimmunity and Inflammation at La Jolla Institute for Immunology. He has conducted pioneering research on the tumor necrosis factor (TNF) superfamily, focusing on understanding the functions and signaling pathways of these proteins in immune regulation, inflammation, and the immune response to cancer, including studies of LIGHT and its receptors and their potential contribution to several inflammatory diseases including asthma. Edelson’s work has focused on understanding the molecular mechanisms underlying lung inflammation, asthma, COPD as well as identifying new therapeutic targets for lung disease and oncology. He has held a number of leadership positions in both large and small pharma including GSK, Aventis Pharma and J&J.
The Foundation for the National Institutes of Health Adds Two to the Board of Directors
The Foundation for the National Institutes of Health (FNIH), based in North Bethesda, added Jay Bradner and Jim Weiss to the Board of Directors. Bradner is the former President of the Novartis Institutes for Biomedical Research (NIBR) and before that, he was a clinician at the Dana-Farber Cancer Institute and an associate professor at Harvard Medical School. Weiss is the Chairman and Founder of Real Chemistry, which provides data-driven, tech-enabled, integrated marketing communications and commercial and medical solutions for the life science sector. Weiss began his corporate career at Genentech and is now an investor, mentor, and strategic advisor to those looking to innovate at the intersection of technology and life sciences.
Theriva Biologics Appoints Ramon Alemany to Senior Vice President of Discovery
Theriva Biologics named Ramon Alemany as Senior Vice President of Discovery, effective immediately. Alemany will oversee Theriva’s discovery and development pipeline and will continue to serve as Chair of the Scientific Advisory Board. Alemany is head of the Immunotherapy and Virotherapy Group at the ProCURE Program of the Catalan Institute of Oncology and the Oncobell Program of the Biomedical Research Institute of Bellvitge. Alemany’s laboratory has developed unique oncolytic adenoviruses that are highly selective for replication in tumor cells, with modifications for tumor-targeting, tumor stroma degradation, evasion of neutralizing antibodies, and promotion of tumor immunogenicity.
Neil Winterbottom, PhD, Joins Medcura’s Leadership Team
Winterbottom has over 20 years of experience leading research and development teams from concept to commercialization, including teams working on sealants and hemostatic agents. Winterbottom holds a doctorate degree in oral biology and biochemistry from the University of Alberta.
Kristin Judge Joins Emmes as Chief Growth Officer
Judge will lead business development and all sales support activities, including inside sales and proposal development. She will support both public sector and biopharma groups.Before joining Emmes, Judge was the head of CRO and clinical data management systems sales at Veeva Systems, leading a global team responsible for more than 200 customers across both the CRO and biotech sectors.
- About the Author
- Latest Posts
Sarah Ellinwood is BioBuzz’s Managing Editor. A scientist by training and a science communicator at heart, Sarah specializes in making complex concepts understandable, engaging, and exciting. She received her Ph.D. in molecular and cellular biology with a focus in infectious disease immunology from the University of Maryland and is passionate about all things related to scicomm, peer mentorship, and women in STEM.