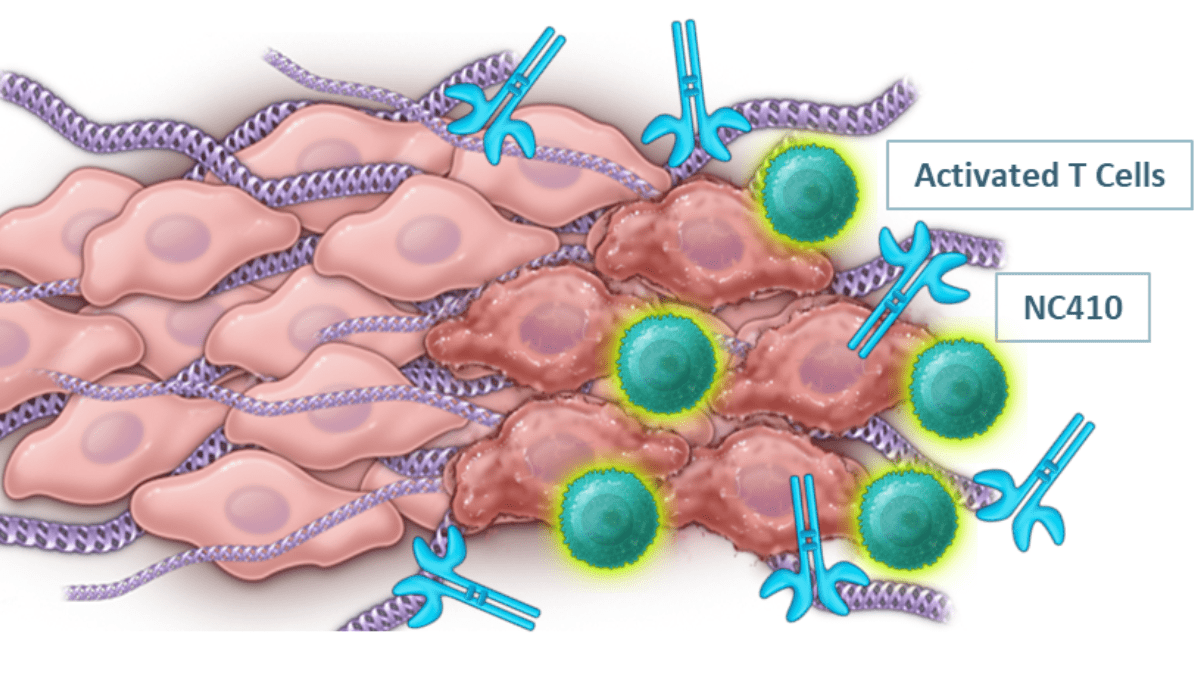
NC410 alleviates immunosuppression, remodels collagen to increase T cell infiltration (SOURCE: NextCure)
How NextCure Leverages the Unique Biology of LAIR to Treat Different Cancers
By Sarah Ellinwood
April 18, 2023
Every year we’re seeing more innovations in cancer therapy, with the market continuing to rise. What used to be only treatable with noxious chemotherapy and radiation regimens now has the potential to be treated with personalized cell therapies and checkpoint inhibitors that harness the patient’s own immune system to fight back.
Checkpoint inhibitors, notably the well-studied PD-1 and PD-L1 inhibitors, have had a major impact on prolonging the lives of cancer patients, some for many years. We’ve come to find, though, that these therapies have limitations. Responses are often short-lived as the tumor adapts to avoid elimination following treatment, or the cancer is not treatable at all with these agents. In the cancer field we can never be confident that we’ve found the cure, as we know that cancers are highly adaptable and tricky beasts to control. We always need to be on the lookout for the next target and the next technique to try and stay a step ahead.
NextCure (Nasdaq: NXTC) is a growing clinical-stage biotechnology company that is working tirelessly to uncover and interrogate new targets and create first-in-class immunomedicines to treat cancer and other inflammatory diseases. The company has an impressive pipeline comprised of several assets, including two that target previously untapped molecular mechanisms driven by the LAIR pathway.
Why Care About LAIR?
Like PD-1/PDL-1, the LAIR signaling pathway is an immune checkpoint that helps regulate the body’s immune response. LAIR-1 is a membrane-bound protein expressed on leukocytes that binds to collagen and other similar proteins with collagen-like structures, called ligands, to suppress immune cell activity. But that isn’t what makes it special and unique. Lots of checkpoint molecules help regulate T cells and other immune cells. The LAIR pathway is special because of how it’s regulated, and how it functions differently depending on the disease.
The activity of LAIR-1 is regulated by a key player called LAIR-2. This protein is similar to LAIR-1 except for two important factors: 1) it’s soluble and “floating” in tissue, or present in blood and other body fluids, instead of being bound to a cell, and 2) it has a much higher affinity for collagen, meaning that it binds tighter to LAIR-1 ligands than LAIR-1 itself. So, LAIR-2 outcompetes LAIR-1 for collagen binding to prevent immunosuppressive signaling. This maintains the balance between suppressing immune cells and risking infection versus hyper-stimulating the immune response too much, such that it leads to inadvertent and undesirable damage.
Like many insidious diseases, cancer tips this balance in its favor.
The microenvironment in many solid tumors is a mesh of highly upregulated and dysregulated collagen. This not only creates a physical barrier against immune cells, but also suppresses immune cells expressing LAIR-1. Naturally occurring LAIR-2 levels are not high enough in the tumor microenvironment to out-compete LAIR-1-mediated suppression. For patients treated with a solo PD-1 or PD-L1 inhibitor, this means T cells that are activated are quickly shut back down in the collagen-rich tumor. Further complicating things, PD-1/PD-L1 therapy can actually promote collagen deposition, exacerbating the very problem that it’s trying to solve. Because of this, patients with higher levels of collagen have an overall worse prognosis and are more likely to develop resistance to PD-1/PD-L1-targeting antibody therapy.
NC410 Opens the Lock
Through understanding the LAIR pathway biology, the NextCure scientific team saw an opportunity. Why not create a LAIR-2-like molecule, a LAIR-2 fusion protein to mimic natural LAIR-2 activity as a novel cancer therapy? The LAIR-2 fusion protein acts as a supplement and collagen-binding decoy to restore immune activity.
Enter NC410.
The LAIR-2 fusion protein is comprised of two LAIR-2 molecules fused to an Fc domain. This molecule serves a one-two punch by both blocking LAIR-1-mediated T cell suppression and remodeling collagen so that T cells can infiltrate and attack the tumor.

NC410 has shown promise in preclinical models and is currently being evaluated in a Phase 1 trial in combination with pembrolizumab in patients with PD-1 resistant/refractory and naive (MSS/MSI-L) patients. So far, data have shown NC410 to be safe and well-tolerated. The Phase 1b/2 trial was initiated in October 2022. A Phase 1b update is expected mid-2023.
“Drug development is a lot like cooking, especially in combination trials. You need to pick spices out of the cabinet that complement each other in the dish,” said Dr. Tim Mayer, Chief Operating Officer at NextCure, in an interview with BioBuzz. “Combining NC410 with pembrolizumab just makes sense – NC410 enables activated T cells to pass through the collagen barrier and ensures that they remain active as they navigate through. It overcomes a lot of the issues we see with solo PD-1 inhibitors.”
NC525 – A Potential New Way to Treat Leukemia
Emerging and compelling data have shown the LAIR signaling pathway may play an even greater role in cancer than once believed, and LAIR-1 signaling affects more than immune regulation.
Especially in leukemia.
Leukemic stem cells strongly express LAIR-1. These same cells also utilize aberrant signaling pathways called mTOR and MAP kinase signaling, to promote their own survival also referred to as self-renewal. Studies have shown that LAIR-1 ties into this self-renewal cascade. However, if the LAIR-1 signal is too strong the leukemic cells will no longer proliferate and will undergo cell death.
This triggering of potent signaling in leukemic cells is precisely what the NC525 agonist antibody does.
NC525 is a monoclonal antibody that binds and crosslinks LAIR-1 with much greater affinity than its natural ligands collagen, delivering a potent signal to leukemic stem cells to suppress self-renewal and instead initiate a type of cell death called apoptosis. Critically, NC525 does not trigger such signaling on healthy stem cells and leukocytes (where mTOR and MAPK pathways are behaving normally), and only triggers cell death in the abnormally dividing leukemia cells.
“Many current AML therapies are toxic to patients. Further, they don’t target the self-renewing cancerous stem cells, meaning there is a chance that the patient will relapse,” said Dr. Mayer. “In preclinical studies, NC525 has been shown to promote killing of AML blast cells and leukemic stem cells while sparing normal hematopoietic stem cells, allowing the normal stem cells to repopulate the bloodstream.”
Phase 1 studies have recently been initiated.
Thinking Big Picture
Many drug discoverers rely on heavy -omics based approaches, sequencing the DNA and RNA of tumors to see which gene expression levels are up or down or if genes are mutated and affecting signaling pathways in the tumor microenvironment, and from there developing drugs to target accordingly. While this has certainly yielded results, Dr. Mayer emphasizes the importance of a broader approach, albeit more complex.
“Cancer isn’t just about overexpression or underexpression – it’s also about how proteins and cell structures communicate with each other,” he said. “While the connection between cancer and the LAIR pathway was initially discovered by our collaborators, NextCure’s FIND-IO™ platform was built for exactly that purpose – to better understand how protein interactions and functions play a role in cancer so we can identify more meaningful targets.”
“The LAIR-1/LAIR-2 story also emphasizes the importance of communication across scientific disciplines as well,” Dr. Mayer added. “Cancer biologists aren’t necessarily experts in collagen biology, so they might miss seeing this important opportunity. Likewise, wound-healing experts aren’t necessarily applying their knowledge regarding this biology to the tumor microenvironment.”
“It’s so important that NextCure’s scientific team has a diversity of training – so that we can bring new and impactful therapies to patients faster.”
Want to be a part of the NextCure scientific team? Check out their open positions
- About the Author
- Latest Posts
Sarah Ellinwood is BioBuzz’s Managing Editor. A scientist by training and a science communicator at heart, Sarah specializes in making complex concepts understandable, engaging, and exciting. She received her Ph.D. in molecular and cellular biology with a focus in infectious disease immunology from the University of Maryland and is passionate about all things related to scicomm, peer mentorship, and women in STEM.









