Kerecis Donates Fish Skin Burn Treatment to Maui Fire Victims
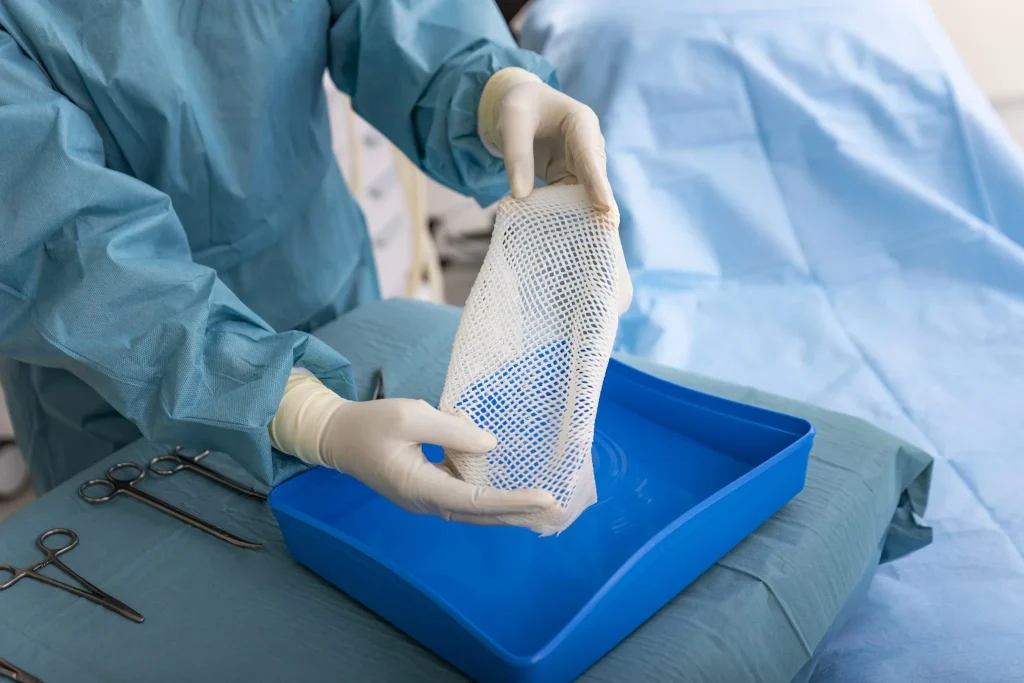
The company’s GraftGuide product is made from the skin of Atlantic cod. The unique FDA-approved wound care treatment is used to regenerate the tissue of burn victims.
By Alex Keown | August 29, 2023
| BioBuzz has been connecting the life science workforce since 2009. We’ve built an expansive community in the Mid-Atlantic with a national readership that spans from Massachusettes to Florida, and New York to California. For our next chapter, we’re building a proprietary talent logistics model to help employers source and hire life science talent. Learn more. |
The wildfires that swept across Maui took the lives of 114 people, and there are still more than 1,000 people unaccounted for. An additional untold number of people were injured by the fires, some likely to have sustained significant burns. Some of those burn victims will likely benefit from a unique fish-skin therapeutic donated to medical facilities and the Straub Medical Center Burn Unit in Honolulu.
The fish-skin graft, marketed by Kerecis, which has its U.S. headquarters in Virginia, promotes rapid healing and skin regeneration when used to treat burn victims. First authorized by the FDA in 2013, Kerecis’ GraftGuide retains a similar porosity and elasticity to human skin. Made from Icelandic cod, the skin treatment has no known risk of viral or prion disease transfer, which means the body is not likely to reject the implant.
Kerecis is one of a number of regenerative medicine companies and organizations located within the BioHealth Capital Region. Regional companies include RegenMD, which focuses on the development of regenerative treatments to alleviate pain and improve functionality. In Virginia, the Joint Regeneration Group of Virginia specializes in treating osteoarthritis through a new, non-surgical therapy that combines cutting-edge, regenerative medicine with custom-fitted orthotics to relieve joint pain and avoid or delay surgery. The University of Maryland School of Medicine operates a Center for Stem Cell Biology and Regenerative Medicine. The university program is focused on developing new treatments and preventive approaches, based on stem cell technology.
Chris Harte, Kerecis’s Chief Operating Officer for North America, told BioBuzz that after seeing the devastation caused by the fires, the company was compelled to send its regenerative medicine product to Maui. It will be a long time before the Hawaiian island and its residents recover from the fire, and Kerecis wants to do its part to aid in the humanitarian effort, he said.
“We’ll send as much [GraftGuide] as they want and need. We want to help the burn victims heal as quickly and completely as possible,” Harte said.
Harte and other members of the Kerecis team are uniquely familiar with the part of Maui most impacted by the fires. Each year, a number of Kerecis employees attend the Boswick Burn and Wound Care Symposium held in Maui. Attending the conference gave Harte an appreciation for the island. Harte recalled a fond memory of sport fishing out of Lahaina, the historic district most impacted by the fires.
“It was such a beautiful place; it was my favorite part of Maui. Now it looks like a nuclear bomb went off there,” he said.
Kerecis has the distinction of being the only FDA-approved skin graft made from fish skin. Most tissue-transplant products are based on tissues of human and porcine origin, but Harte said these are not ideal substitutes. That’s primarily due to the heavy processing required to eliminate the risk of disease transmission. He said the anti-viral treatment those skin products undergo removes most of the natural components, which makes it dissimilar to human skin.
Kerecis’ GraftGuide is made from the intact skin of Atlantic cod caught from a sustainable fish stock in Iceland. The fish-skin treatment only needs mild processing, which allows it to retain porosity similar to human skin, Harte said. The Kerecis product has several advantages over other products made from human or porcine membranes. This is primarily due to the lack of viral transfer concerns and the product’s economic and environmental sustainability.
Harte said the company’s manufacturing center in Iceland is blocks away from a fish processing center. Traditionally, the fish skins are tossed out after the meat is extracted. Harte said Kerecis takes those skins instead and converts them into a medical product.
Alongside its burn treatment product, Kerecis also manufactures other wound care treatments using the same fish skin technology. The Kerecis products are used to treat burn wounds and other complex acute and chronic wounds including diabetic, venous, trauma, and surgical injuries.
“It’s an FDA-approved product that does require some training,” Harte said. He noted that there are some doctors in Hawaii who are already trained in the use of GraftGuide.
Maui isn’t the only place where Kerecis has sent its fish skin products to serve humanitarian efforts. The company also sent its medical devices to the front lines of the war in Ukraine, as well as to treatment centers on the frontlines of the ongoing conflict between Armenia and Azerbaijan. Kerecis also sent its burn product to aid victims of the 2019 volcano eruption in New Zealand. In that incident, approximately 31 people were injured with burns over 30% of their body, according to BBC reports.
“One of our core values is compassion. This is not an empty word. We are living that core value of compassion. Where we can see and do good, that’s where we’re going to try and go,” he said. Qualified medical personnel wanting to get fish-skin burn-treatment products for their patients should call 703-287-8752 or email [email protected]. Kerecis is dispatching a specialist to train doctors who would be using the product for the first time.







