Novan Inc. Sets Sights on Approval of Topical Molluscum Contagiosum Treatment
By Alex Keown
March 9, 2023
Novan, Inc. is one step closer to securing the potential greenlight of the first FDA-approved prescription product for molluscum contagiosum following the regulatory agency’s acceptance of the company’s New Drug Application.
Durham-based Novan submitted its NDA for berdazimer gel, 10.3%, a potential first-in-class topical gel, in January. The FDA set a PDUFA date of Jan. 5, 2024.
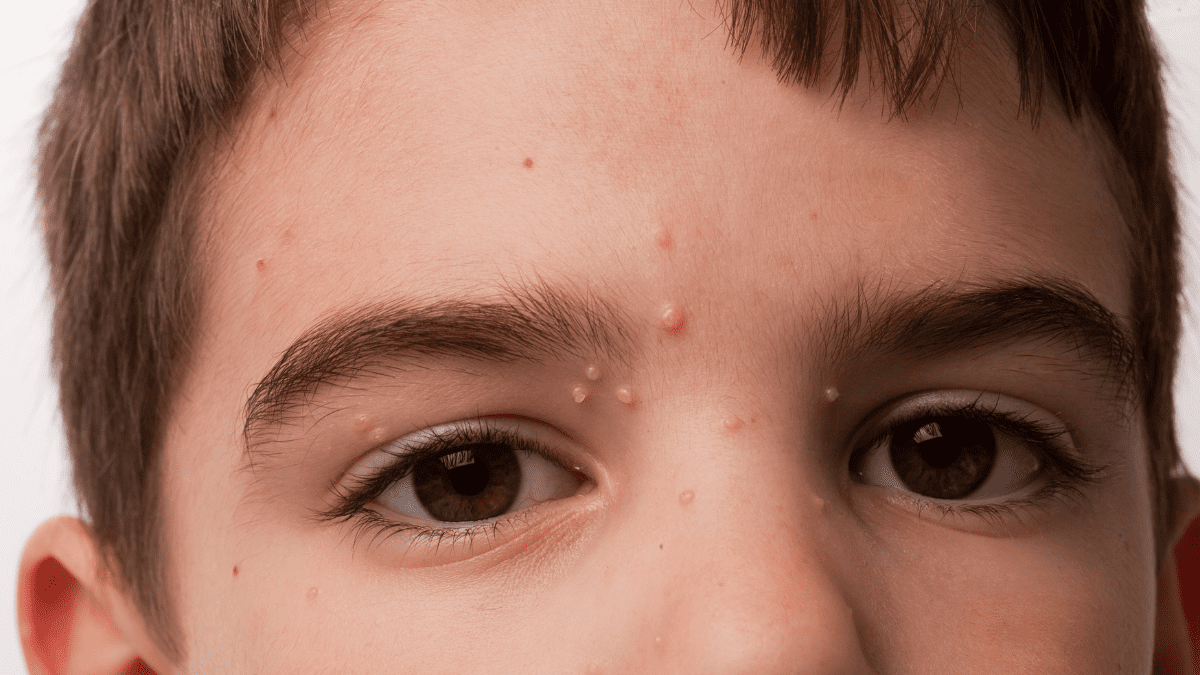
Paula Brown Stafford, president and chief executive officer of Novan, expressed excitement that Novan could receive approval for berdazimer gel, 10.3% in 10 months or less. Approval would mark the first topical treatment for molluscum contagiosum, a common and contagious viral skin infection caused by the molluscipoxvirus.
“We have the opportunity to have an approved product in 10 months for an unmet medical need for children,” Brown Stafford said.
Molluscum contagiosum affects more than six million people in the U.S., the majority of them children. The infection typically causes between 10 and 30 lesions over a patient’s body. The majority of the lesions typically appear on the trunk and limbs, although lesions on the face are not uncommon, Brown Stafford said. In some cases, the number of lesions can be significantly higher. The infection can last for months, or years at a time, she added.
Currently-available treatments are typically uncomfortable in-office visits that involve cryotherapy, laser therapy or curettage, which involves piercing the lesion and scraping out the caseous material, according to the Centers for Disease Control. Because of the limited treatment options. It is estimated that approximately 70% of molluscum patients go untreated.
With berdazimer gel, 10.3%, patients will be able to self-administer the treatment. Brown Stafford said it’s as simple as dabbing a little bit of the gel on the lesion. Clinical data showed the spot treatment was able to “reduce and decrease and potentially clear all of the lesions within 12 weeks or less,” she said. That’s a significantly short amount of time when compared to how long the virus can remain active within the body.
“The ability to use the gel once-per-day instead of having to undergo one of those procedures is an appealing option for patients and treating physicians,” Brown Stafford said.
The drug’s active ingredient is berdazimer sodium, a new chemical entity that releases nitric oxide and has anti-viral activity. Brown Stafford noted berdazimer sodium has additional applications in other dermatological areas, including acne, genital warts and treatment of nail fungus. The concentration of berdazimer is adjusted to suit the different indications, Brown Stafford noted.
The NDA for berdazimer gel, 10.3% is based on data from the pivotal Phase III B-SIMPLE4 clinical trial. In that study, berdazimer gel demonstrated “highly statistically significant improvement” in treating the lesions. The trial included the largest cohort of molluscum patients ever studied, according to the company. Data from the study were published in JAMA Dermatology. Berdazimer gel, 10.3% was found to be well tolerated with mild application site pain and mild to moderate erythema reported as the most common adverse events.
Novan isn’t the only company gunning to be the first with an FDA-approved treatment for molluscum contagiosum. In January, Pennsylvania-based Verrica re-submitted its NDA for VP-102, a drug-device combination product that contains a formulation of cantharidin. The FDA issued a Complete Response Letter to Verrica in May 2022 after deficiencies at the company’s contract manufacturing organization were discovered.
As Novan awaits the 2024 PDUFA date, Brown Stafford said the company will focus on manufacturing berdazimer sodium at its Durham manufacturing facility to prepare for an anticipated commercial launch. The company will also talk with payers and providers and conduct medical outreach ahead of potential approval.






