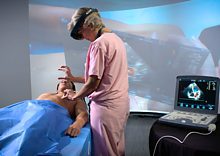
With $5 million from the U.S. National Science Foundation (NSF) and technology titans including Google, Microsoft and Meta (formerly known as Facebook), a trio of academic institutions are collaborating with industry and the federal government to develop, test and certify XR technologies in medicine and health care.These medical examples of extended reality (XR)—the umbrella term used for technology based in virtual and augmented reality or other immersive media—are already being prototyped or tested in clinical trials. But its widespread use in hospitals and other health care settings is currently hampered by technical challenges and sparse regulatory guidelines.
Now, with $5 million from the U.S. National Science Foundation (NSF) and technology titans including Google, Microsoft and Meta (formerly known as Facebook), a trio of academic institutions are collaborating with industry and the federal government to develop, test and certify XR technologies in medicine and health care.
The new Center for Medical Innovations in Extended Reality, known as MIXR, joins University of Maryland computer scientists and engineers with physicians and clinicians at the University of Maryland School of Medicine in Baltimore and the University of Michigan to improve medical training, patient management and health care outcomes across all areas of…
Bringing Health Care’s Vision of Tomorrow Into Focus | UMIACS