Novavax Searching for New “SuperNova” Talent, Hosts Virtual Recruiting Event
In an incredibly short time, Gaithersburg, Maryland’s Novavax has advanced its SARS-CoV-2 vaccine candidate into Phase III trials and received FDA fast track designation, putting its vaccine very close to potential FDA approval. There has been a herculean effort underway by Novavax and other companies to deliver desperately needed pandemic protection to people across the globe.
This remarkable push, assisted by Operation Warp Speed, has compressed a vaccine development timeline that normally takes eight to ten years into eight months, which has required Novavax to hire new talent to advance its vaccine and other pipeline products.
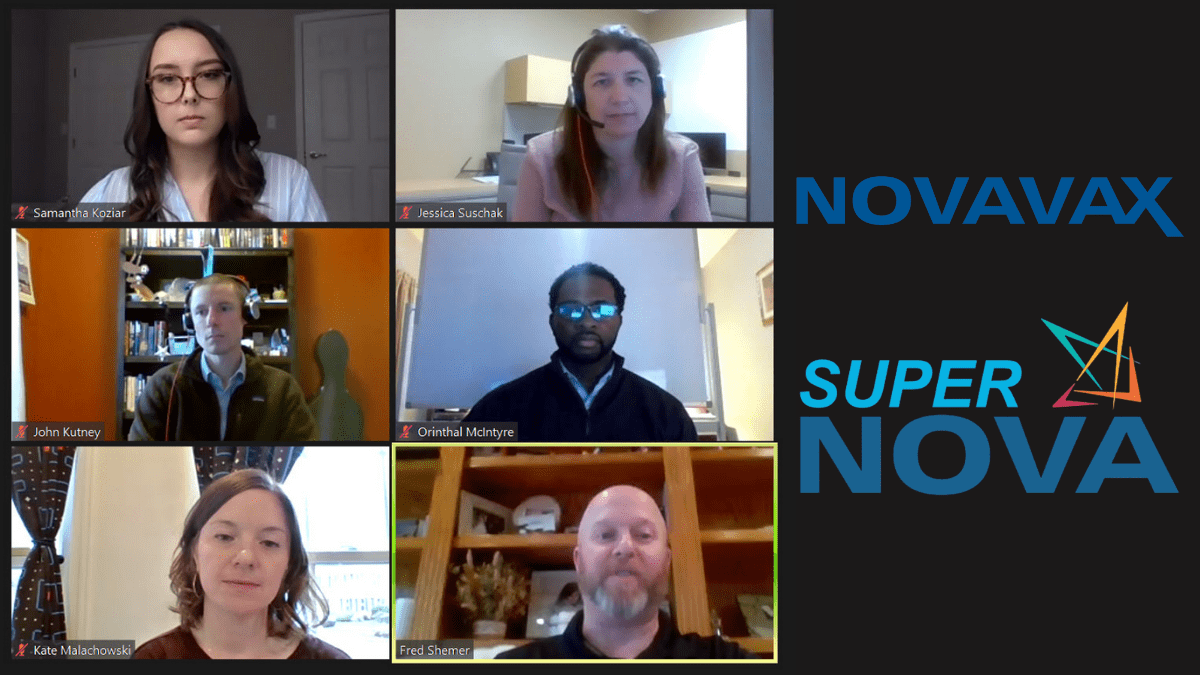
Novavax recently held a Virtual Recruiting Event (VRE) where life science job seekers had the chance to hear from Novavax team members and participate in a robust Q&A session. Novavax participants in the event included: Stephanie Major, Executive Director, Talent Acquisition; Fred Shemer, Vice President, Quality Assurance; John Kutney, Senior Director, Manufacturing; Kate Malachowski, Associate Director, MS&T; Jessica Suschak, Senior Manager, CMC Analytics; Orinthal McIntyre, Senior Quality Assurance Manager; Samantha Koziar, QA Training Specialist II; and Arthur Zavadsky, Manager, Talent Acquisition.
Fred Shemer, VP, Quality Assurance and Compliance, kicked off the event by stating, “Over the last six years I’ve been part of an exciting time at Novavax where the company has developed multiple vaccines candidates for RSV, influenza, and COVID. With the strong data we have we have been able to obtain $2B in funding from SEPI and Operation Warp Speed to develop a pandemic vaccine.”
“This funding has allowed our SuperNova population to grow exponentially as we push to meet the exceptionally aggressive timeline for the development of our COVID-19 vaccine,” he added. “We’ve dosed over 20,000 volunteers in our COVID trials to date…these accomplishments are only possible because of our greatest resource, our SuperNovas. Since the beginning of 2020, the QA group has grown to approximately 40 SuperNovas and we have openings for another 15 quality professionals.”
This is a testament to Novavax’s people and the strong, vibrant workplace culture it has built, evolved, and sustained over the years called SuperNova. Novavax’s SuperNovas are skilled, driven, and accomplished people that work in an environment that empowers them to do what they do best and to do what they love most. The SuperNova mission has been crystallized and taken on new meaning during the fight against the coronavirus pandemic.
“I am a SuperNova because I want to make a positive difference in the lives of our patients,” shared Shemer.
Novavax’s current openings include but are not limited to:
- Associate Director, Quality Assurance
- Sr Manager/Manager, QA
- QA Specialist IV, Operations
- Manager, Tech Transfer
- Tech Transfer Lead
- Tech Transfer Lead, Drug Substance Operation
- Director, MS&T
- MS&T Engineer
In late February 2020, Novavax announced that its COVID-19 vaccine candidate had made progress in animal models. Just a few months later, the company secured $388M in funding from the Coalition for Epidemic Preparedness and Innovation (CEPI). In June and July, Novavax received an influx of funding from the Department of Defense (DoD) and Operation Warp Speed, the latter providing $1.6B in vaccine funding. Just six months later in August 2020, Novavax announced positive Phase I data, which was followed by the start of Phase II clinical trials that same month. In September, Novavax’s Phase III trials in the U.K. commenced and the company received Fast Track Designation from the FDA in early November of this year.
“It seems hard to imagine it’s only been 10 or 11 months since COVID-19 arrived,” stated John Kutney, Senior Director, Manufacturing.
“We’re solving multiple key challenges to launch a new product. Among those include developing our process, scaling it to larger manufacturing sizes, transferring it to manufacturing sites around the world, developing a global supply chain, and developing a regulatory strategy for emergency use. These steps would normally take years, but here we are on the cusp of getting Phase III results in less than a year. We’ve been building the plane while flying it,” he added.
“This is the most gratifying work I’ve done. I am a SuperNova because I feel that I have the opportunity to impact human health worldwide everyday,” he shared.
A key to Novavax’s vaccine success to date is effective tech transfer, which is essentially taking a highly technical manufacturing process and teaching another organization how to replicate this process. Effective and efficient tech transfer to Novavax’s global manufacturing sites requires collaboration among a number of functions at the company, including Manufacturing, Quality, MS&T, Analytics, and Human Resources. As part of this massive, global tech transfer effort, team members across the company have the opportunity to solve new problems every day that will make a tangible public health difference.
“MS&T is critical because of the global nature of our operation. With multiple drug substance sites all supporting our vaccine, we need to ensure they have a control strategy at their facility that will produce comparable material. We’re looking for people who are flexible and able to respond to challenges; people that can understand and quantify a problem and identify root causes and move forward with solutions,” stated Kate Malachowski, Associate Director, MS&T.
“I am a SuperNova because I like to be challenged at work every day,” she added.
In just seven months, Novavax scaled up and expanded its manufacturing and distribution network across the U.S. and the globe. With its global network now in place, the important work of effectively transferring the vaccine technology to Novavax’s new vaccine partners while protecting Quality Control (QC) has begun in earnest. Vaccine development and manufacturing is a delicate, complex process even when completed in-house within a single company. Tech transfer and QC become even more complex when coordinating with a global development and manufacturing network.
Orenthal Orinthal McIntyre, Senior Quality Assurance Manager, shared, “We have our hands in a lot of assets when it comes to GMP manufacturing. We’re looking for candidates that are experienced, quality leaders that have a strong technical background; we’re searching for hard, independent workers that are able to think out-of-the-box and that work well within a team.”
“We are a well-oiled machine and support one another and we never give up. There is no back down at Novavax and that’s why I am a SuperNova,” he added.
Novavax’s recent growth has also brought several former Novavax employees back into the family. These so-called “Boomerang” employees are a testament to the company’s strong, vibrant culture and the exciting, meaningful work that it supports.
Shemer noted that three of the virtual recruiting event participants were Boomerang employees, including McIntyre, Malachowski, and Jessica Suschak, Senior Manager, CMC Analytics.
Suschak stated, “My journey at Novavax has been extremely rewarding. I started as a bench scientist in the analytical development department and became heavily involved in our RSV and NanoFlu programs. I eventually moved on to an AD scientist role at another company that interestingly supported the NanoFlu program. During that time I realized I missed the camaraderie of my Novavax colleagues and the fantastic science we were doing. Luckily for me, I was able to find another position back at Novavax. My decision to rejoin was simple: great science, great people, and great benefits.”
“I am a SuperNova because I get to play a small part in providing vaccines that will save lives around the world,” she added.
“We’ve had multiple employees leave us and come back and that’s a testament to our level of teamwork and culture,” stated Shemer.
Because of the pandemic, Novavax’s recruiting and hiring has gone nearly 100% virtual. The company’s recent virtual recruiting event is part of Novavax’s virtual recruiting strategy.
“We’re trying to take as many precautions as we can during these times. We’ve turned to virtual interviews. We want to make sure we keep everyone safe,” shared Stephanie Major, Executive Director, Talent Acquisition.
“We’ve gone all virtual and now conduct all stages of interviews using Microsoft Teams. This will continue into 2021 where we anticipate more growth and hiring,” stated Arthur Zavadsky, Manager, Talent Acquisition.
Novavax is searching for talented people with technical knowledge, versatility, flexibility of approach, compassion, attention to detail, creativity, a collaborative spirit, and a problem-solving mentality.
“As we move from a clinical organization to a commercial one, we’re looking for talent that has the capacity to learn. We’re looking to bring people in with technical experience that are willing to develop alongside our business, culture, and teams,” shared Shemer.
If you’re interested in becoming a SuperNova and collaborating on projects that will help end the pandemic and positively impact global public health, visit the Novavax career page to learn more about their job openings.
- About the Author
- Latest Posts
Steve brings nearly twenty years of experience in marketing and content creation to the WorkForce Genetics team. He loves writing engaging content and working with partners, companies, and individuals to share their unique stories and showcase their work. Steve holds a BA in English from Providence College and an MA in American Literature from Montclair State University. He lives in Frederick, Maryland with his wife, two sons, and the family dog.